Abstract
Introduction: Increased numbers of plasmacytoid dendritic cells (pDC) in the bone marrow graft improve clinical outcomes with decreased graft-versus-host disease (GvHD) and improved survival in previous pre-clinical murine experiments (Lu Y, Blood. 2012;119:1075-1085) and in clinic data from BMT CTN 201 (Waller JCO 2014; 32:2365-72). The mechanism by which donor pDC limit severe GvHD and improve survival after allo-BMT is unknown. We have previously described higher levels of donor T cell activation and expansion (measured as bio-luminescent radiance) among recipients of VIP-KO pDC (Li, J.X Blood 2016 128:2154). We hypothesize that blocking VIP signaling augment the magnitude of T cell activation and expansion, and that modulating the VIP pathway may be a potential therapeutic target for regulating GvHD in allo-HSCT.
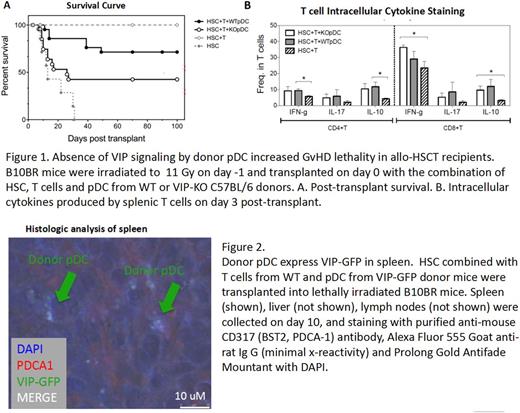
Methods:We used a MHC mismatched C57BL/6 àB10BR allo-BMT model, transplanting purified populations of FACS sorted Lin-Sca-1+C-kit+ (LSK) stem cells combined with FACS purified BM pDC and MACS purified splenic T cells. Lethally irradiated B10BR recipients received 5,000 HSC, 50,000 pDC from wild type (WT) or VIP-KO donor and 1×106 T cells from WT C57BL6 donor mice. Survival and GvHD were measured based on fur texture, posture, activity, skin integrity and weight body loss. To test VIP production by pDC cells, we purified donor pDC from VIP-GFP C57 BL/6 mice, in which GFP transcription is under the control of the VIP gene promotor, and transplanted them into lethally irradiated MHC mis-matched B10.BR recipients. VIP expression in mice receiving pDC cells from a VIP-GFP transgenic donor was detected by fluorescence confocal microscopy. Immune polarization of donor T cells was accessed by intracellular cytokine staining and flow cytometry. Mice transplanted with VIP-GFP pDC cells were sacrificed on D10 and spleens were collected in OCT.
Results: Survival of recipients receiving HSC, T cells and WT pDC was 71% on day100 post-transplant while survival of those receiving VIP-KO pDC was 31% (Fig 1A). Splenocytes were obtained from recipients on day 3 post-transplant. IFN-γexpression was higher and IL-10 epression was significantly lower in donor CD8+ T cells in mice receiving VIP-KO pDC compared with WT pDC (Fig 1B). Confocal microscopy showed VIP expression in donor pDC among recipients of VIP-GFP pDC (Fig 2)
Conclusion:These data indicated VIP signaling occurs via interactions between donor T cells and donor pDC. Local production of VIP inhibits activation and Th1 immune polarization of donor T cells. In murine allo-BMT, absence of VIP expression in pDC leads to increased T cell activation, expansion, allo-reactivity and Th1 polarization resulting in worse survival. Thus VIP is a critical pathway in regulating GvHD and GvL action of donor T cells and an attractive pharmacological target for GvHD prevention.
Waller: AMGEN: Consultancy; PRA: Consultancy; Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Celldex: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Helocyte: Consultancy; Cerus: Equity Ownership; National Institutes of Health: Research Funding; Chimerix: Equity Ownership; Coulter Foundation: Research Funding; Katz Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal